Ocean acidification – a price our ocean pays for soaking up carbon
 We depend on our ocean to make our world livable. The ocean absorbs and distributes heat and CO2, protecting us from a runaway greenhouse effect that would turn Earth into another uber-hot planet like Venus.
We depend on our ocean to make our world livable. The ocean absorbs and distributes heat and CO2, protecting us from a runaway greenhouse effect that would turn Earth into another uber-hot planet like Venus.
But the ocean is changing along with our climate, and it’s affecting the ocean’s capacity to store carbon as well as its chemistry. This is affecting the life cycles of many marine organisms, particularly those at the lower end of the food chain.
A Question of CO2 Balance
The ocean is the planet’s second biggest reservoir of carbon (after rocks). Oceanographers estimate the ocean has absorbed about 30% of the CO2 pumped into the atmosphere since the industrial era began. The current rate is estimated at 22 million tons per day.
The oceans are able to hold more carbon than the atmosphere because the CO2 that the ocean absorbs is dissolved into non-gaseous, more stable forms. Scientists refer to this as the “carbon sink.”
The oceans also exchange CO2 by releasing it into the atmosphere, generally in tropical waters. Overall, however, the oceans are now absorbing more CO2 than they release.
Bottom line, there’s more CO2 going into the ocean from our atmosphere. This imbalance seems like a good thing for our climate, but the uptake has a downside – ocean acidification.
In the past 200 years, ocean water has become around 30 percent more acidic—that’s faster than any known change in ocean chemistry in many millions of years, according to scientists.
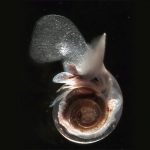 Ocean acidification has been shown to be particularly damaging to the many organisms that use calcium carbonate to build protective shells, including some phytoplankton and invertebrates such as corals, sponges, marine worms, mollusks, and crustaceans.
Ocean acidification has been shown to be particularly damaging to the many organisms that use calcium carbonate to build protective shells, including some phytoplankton and invertebrates such as corals, sponges, marine worms, mollusks, and crustaceans.
Increased acidity makes it harder for these marine organisms to form skeletons or shells, called “calcifying.” When these species decline, it can endanger the entire food chain.
There is also concern that if seawater grows acidic enough, it may become corrosive to shells and interfere with animal and fish reproduction, respiration and behavior.
Ocean acidification isn’t bad news for all sea life, and some species can adapt better than others. Unfortunately, the lower pH seems to be helpful to harmful or invasive species, such as “killer” algae, jellyfish, and invasive mollusks.
Evidence suggests that rapidly acidifying oceans may have played a major role in previous mass extinctions of marine life.
The Chemistry of Ocean Acidification
The pH of surface seawater has fallen from 8.2 to 8.1 in a couple hundred years, after remaining constant for millions of years.
This .1 ph change may seem insignificant, but it translates to a 30 percent rise in acidity. That’s due to the logarithmic nature of the pH scale, like the Richter scale for earthquakes. A change of one pH unit equals a ten-fold change in hydrogen ion concentration (more hydrogen=more acidic).
To put it into perspective, a slight change in the pH of human blood can cause “acidosis’ and seizures, comas, and even death. Some aquatic life is equally as sensitive. This can also happen to aquatic life.
The ocean itself is not actually acidic. It’s more accurate to say the ocean is becoming less alkaline, since the pH is greater than 7 (neutral). Even if the term ocean acidification is somewhat alarmist, the downward direction toward acidity is still dramatic.
Carbon moves between the ocean and atmosphere by a process of molecular diffusion. When the atmospheric CO2 gas pressure is higher than the surface ocean, CO2 diffuses or is absorbed into the sea water.
A series of chemical changes break down the CO2 molecules and recombine them with others, resulting in increased acidity.
When water and CO2 mix, they combine to form carbonic acid, a weak acid that gives soda its fizz. Like all acids, carbonic acid releases hydrogen ions which bond with other molecules. Carbonic acid dissolves rapidly and forms hydrogen ions (an acid) and bicarbonate (a base). Seawater contains another base, carbonate ion, which acts like an antacid to neutralize the hydrogen ions, which forms more bicarbonate.
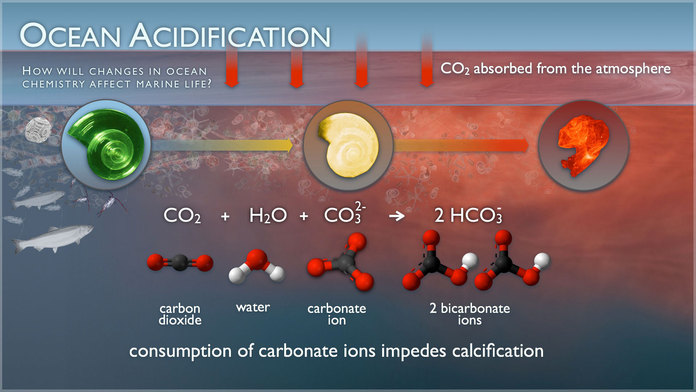
Credit: PMEL Carbon Program
The depletion of carbonate ions is the problem- it causes two calcium carbonate minerals vital for shell-building seawater to becomes less available.
This depletion wasn’t a concern in the past since carbonate ion in the ocean is replenished through the weathering of limestone rock carried by rivers, and by dead animals, such as pteropods. But that process which can take hundreds of years can no longer keep up with the rapid changes from increased CO2 absorption to keep the system in balance.
In addition, an ocean with less carbonate ions has less capacity to absorb more CO2, which increases that other serious CO2 problem- global climate change.
The trend of ocean warming is also a key player in the carbon balance equation. Ocean warming slows the circulation that sinks carbon and mixes carbon from the surface layers with water from deeper levels.
Current Research Frontiers
Many ocean acidification studies have been done somewhat in a vacuum. The challenge for researchers is to measure the effects of ocean acidification throughout the food web.
Ocean acidification is a relatively new and multi-disciplinary research area that encompasses such topics such as chemistry, biology, ecology, paleontology and biogeochemistry. It’s a field that can be quite complex and counterintuitive, such as with the carbonate chemistry, which can make the results confusing to the public and media.
 Evidence for the ecological effects of ocean acidification have largely been based on past geologic history, and from “champagne sites,” locations where vents of volcanic CO2 naturally acidify the water and CO2 bubbles up through the water column.
Evidence for the ecological effects of ocean acidification have largely been based on past geologic history, and from “champagne sites,” locations where vents of volcanic CO2 naturally acidify the water and CO2 bubbles up through the water column.
Researchers are working to better understanding how species adapt to higher-CO2 seawater, both those directly and indirectly affected. Species that live in shallow reefs with more varied conditions may be more adaptable than those who inhabit the open ocean.
Increasingly, studies are showing a clearer link between ocean acidification and the disintegration of key corals. But not all corals are affected.Corals can actually change the pH chemistry of the seawater around them, by building and dissolving their skeletons which pulls and releases calcium carbonate into the seawater.
Ocean Acidification – How You Can Help
Most issues we face with the oceans interconnect with environmental problems on land and in the atmosphere.
- Addressing carbon and nitrogen emissions is important not only for our climate, but also for the oceans. Individuals and communities can help by burning fewer fossil fuels, using more renewable energy and curbing chemical pollution from agricultural and residential runoff.
- Conservation efforts such as restoration of wetlands and reforestation can reduce the ocean’s burden atmospheric uptake of CO2 by enlisting more trees and vegetation.
- Active approaches you can do include contacting policy makers to devote money to research and monitoring, particularly in areas with vulnerable fisheries such as oysters and other shellfish, and volunteering/donating to conservation organizations that study ocean acidification.
RESOURCES:
Acid Test: The Global Challenge of Ocean Acidification
Ocean Acidification: The Chemistry Video Presentation: Ocean’s Acid Test Ocean Faces Mass Extinction – Broad Study
FAQs about ocean acidification – EPOCA
Ocean Acidification News Stream – Ocean Acidification International Coordination Centre (OA-ICC)
Ocean Acidification – Summary for PolicyMakers from
Third Symposium on the Ocean in a High-CO2 World
Rising carbon dioxide levels stunt sea shell growth
Sea Change: Creatives Behaving Strangely


